We did it two weeks ago. Since then we have been carefully watching the progress. And now we can share the news!
Technology, teamwork and abilities to immediately adapt to new conditions are absolutely required for the successful start of a clinical trial during the pandemic. Teams of R-Pharm (innovative company from the TOP-3 largest pharma in the Eurasian Economic Union) and Data Management 365 have a long history of partnership. Together we just broke the record for the quickest launch of a clinical trial.
R-Pharm is known for its high-tech solutions and achievements in innovative healthcare products development. The company could not stand aside the world’s biggest challenge and responded immediately. It became the first EEU company to submit a request and get the regulatory authorization of a COVID-19 related clinical trial. Then Data Management 365 took responsibility for organizing the process of clinical data collection and investigational product assignment. DM 365 was chosen for its proven engineering experience and profound expertise in successfully solving similar tasks.
Task #1. Launch the project under constantly changing circumstances and demanding requirements within an extremely tight timeframe.
Solution. The project was moved into production really quickly. It was built in such a way that allows for multiple amendments and adjustments to be implemented promptly during the study – from a single form update to changing the whole randomization scheme.
Task #2. Provide comfortable working environment for Investigators at clinical sites, since they may face difficulties when
- pulling the source documentation out of the ICU,
- passing essential steps to randomization and drug dispensing inside the ICU,
- authenticating on electronic devices still wearing thick gloves, facial masks, glasses, etc.
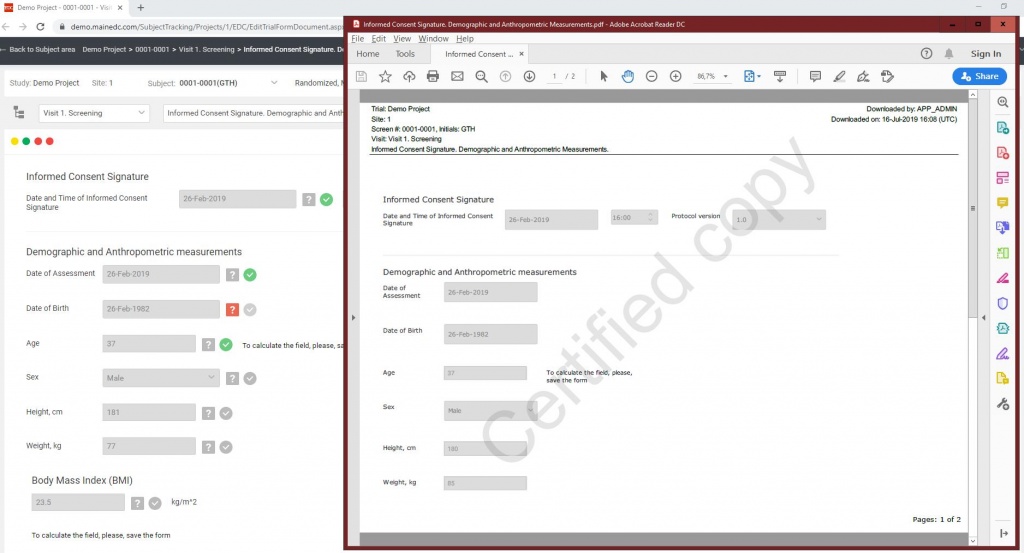
Solution. Data Management 365 developed unique hybrid project design which allowed quick and simple data flow. It takes less than 15 seconds to turn on a tablet, input data and dispense a drug.
Task #3. Ensure remote monitoring of clinical sites for both blinded and unblinded teams.
Solution. Quintessential solution was successfully mounted upon the MainEDC™ COVID-19 Pack (which we released in March 2020). Both teams studied recommendations of FDA, EMA and ACTO, and worked side-by-side through the regulatory, operational and technical limitations.
Task #4. Adаpt to highly raised requirements for subjects safety.
Solution. Adopted solution successfully allows the following:
- flexible notifications and alerts to all significant clinical abnormalities and events including AE/SAE are available for all users,
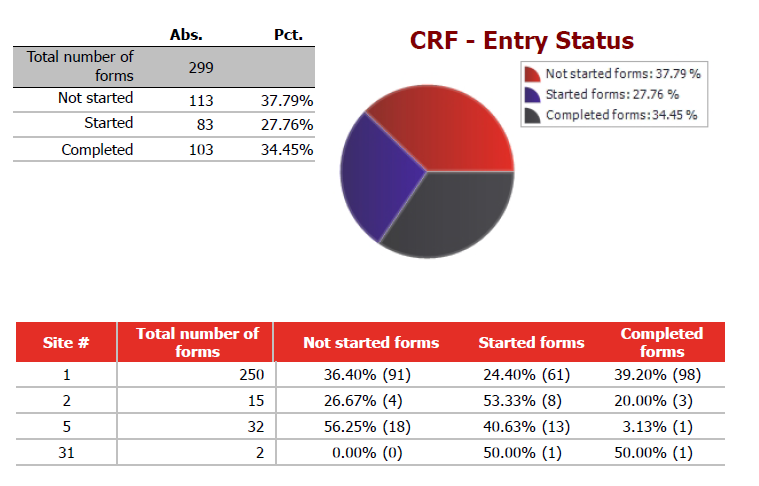
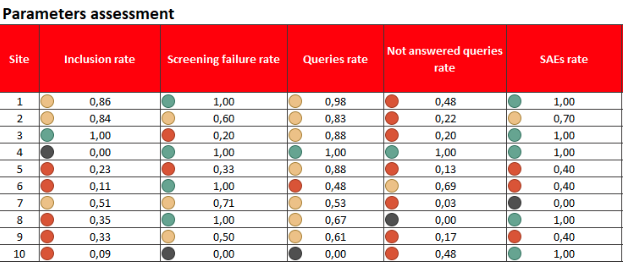
- real-time reporting (e.g. KPI report, medical listings, online dataset exports, etc.) are available for Study management and CRAs.
Task #5. Expedite drug supply and dispensing during the pandemic. It severely complicated these processes with obstacles imposed by different uncertainties, delivery time and even some clinical sites’ inability/refusal to take part in the trial.
Solutions. Data Management 365 developed a unique interface for unblinded users and introduced special procedures for logistics and distribution of the investigational product.
Within this mutual project Data Management 365 and R-Pharm established a new record for the quickest launch of a clinical trial. It only took us 6 days from final protocol to project release on production, including full eCRF, randomization design and drug supply algorithm implementation. Once again, we proved that synergy of highly qualified professionals and innovative engineers in the industry is the key to an effective and efficient clinical trial!
Both teams’ leads commented on the successful results of the launch:
Mikhail Samsonov, СМО at R-Pharm: “COVID-19 clinical trial requires high speed, joint effort and innovative solutions. Moreover, the role of Investigators, their recommendations and focus on quick implementation of data capture tools become crucial for successful high quality study completion addressing emerging patients needs”.
Timur Galimov, CEO at Data Management 365: “I am very excited about the results and our contribution to such a meaningful project. We were pleased to work together with our great partner R-Pharm! Accordingly, we allocated our best resources and technology. As a result, we developed a highly innovative and user-friendly solution, which allows quick launching of new studies to the highest standard of data quality and subjects safety”.
Both companies are now preparing a mutual press-release with more information about the project itself. We hope to share more details with you soon! Please keep an eye on our posts.